Monoclonal Antibodies Facility Design and Simulation
Designing a robust and cost-efficient monoclonal antibodies (mAbs) manufacturing facility is a significant challenge in the life sciences industry due to the difficulty of forecasting product demand and production. By integrating process simulation, coupled with capital and operating cost estimation, one can benefit from an efficient method of evaluating alternatives to find an optimized design.
Common design alternatives listed below provide a sense of the scope of the optimization:
- Throughput, usually defined as the number and size of the production bioreactors, is a key variable. Batch timing and cadence also contribute to throughput modeling. Consideration must also be given for making disparate products concurrently in the same facility.
- Some cell culture processes are strictly batch, while others are semi-batch or perfusion. This will influence the size of the equipment and the media delivery strategy.
- Chromatography and ultrafiltration/diafiltration operations require multiple buffers. These may be produced and delivered for each main product batch entailing many small volume preparations with little buffer storage but may be labor intensive. Alternatively, buffers may be multi-batched (where one buffer batch provides for multiple product batches), or buffer concentrates may be used, which will reduce preparation batches, saving labor. Additionally, central storage and distribution systems for standard buffers, such as various caustic concentrations, are often utilized.
- Buffer storage will be a function of multi-batching and use of concentrates also. This decision will impact the WFI use profile and the design of the utility supply system.
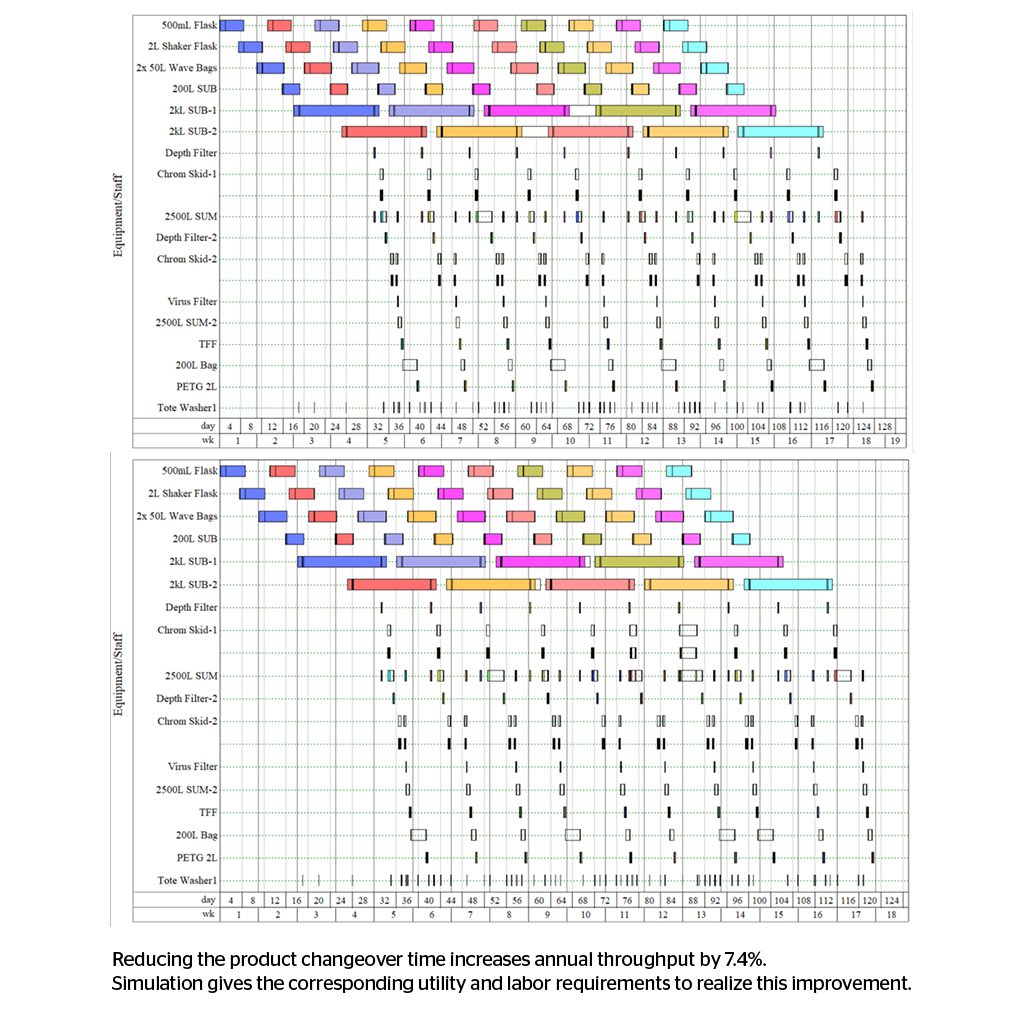
- The location of equipment within separate suites or ballrooms impacts the facility’s cost, affects campaign changeover and hence throughput. Typically, one batch or product is allowed in a suite at one time. The equipment and suite may require more intense cleaning between campaigns which is unproductive time for the facility.
- Stainless steel equipment vs. single-use systems is a critical decision that will impact equipment cost, CIP, and water system design. Disposables will save on several costly elements of the facility but will entail purchasing expensive, single-use consumables, increased labor, increased solid waste, and will require additional staging space. This is evaluated as a transfer of capital cost to operating costs.
- Future needs such as increased titers, additional products, or throughput expansions impose design constraints or demands on the facility.
Each of the above options, and many others, can affect the size of the facility, its configuration and capital and operating costs. They may also impact flexibility, reliability, operability, and throughput.
Process simulation broadly means representing the process, or an abstraction of the process, on the computer, where it can be readily studied. The software tool and granularity of the model depends upon its specific purpose. Adding too much detail slows the development and delays the results. Too little detail and the model may miss essential behaviors of the real-world process. Simulation projects begin with a statement of purpose, and the goals of using this technology. Data collection then begins. The data may originate with querying a data historian, simply interviewing a subject-matter expert, or anything in between. A base case model is then constructed and checked to ensure it performs as expected and appropriately matches historical performance data. .
The base case model can be changed to represent alternatives and compare simulation results against the base case. Design alternatives, operating alternatives, product changes, and process changes can all be studied and compared using simulation-generated metrics. These metrics typically include batch cadence, equipment utilization, utility usage vs. time, staffing requirements, and, ultimately, throughput for a given product slate. Process upsets can be modeled, providing a case for redundant or alternate systems. Capital and operating costs can be calculated from the modeling results. Simulation results can justify or rule-out proposed design changes. Simulation answers the questions: “Do we need a change, and is this proposed change worthwhile?”
The design of such a complex manufacturing facility is complicated given the many objectives: reliable drug substance supply, cost efficiency, cGMP, and regulatory considerations. Process modeling and simulation provide a clear path through the deep, dark forest.